Maltose starch powder was analyzed on SUGAR KS-801 according to Japanese Pharmaceutical Excipients 2013 method. It is necessary for the choice of column to satisfy resolution of maltotriose/maltose of ≥ 1.6 and maltose/glucose of ≥ 4.1. It was confirmed that resolution of maltotriose/maltose was more than 2.0 and that of maltose/glucose was more than 5.0 when they were analyzed using KS-801.
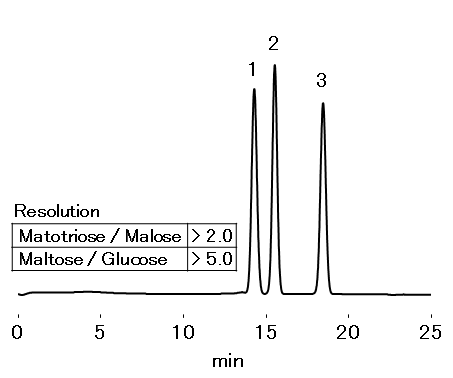
Sample : 5 mg/mL each, 10 µL
1. Maltotriose
2. Maltose
3. Glucose
Column : Shodex SUGAR KS-801 (8.0 mm I.D. x 300 mm) Eluent : H2O Flow rate : 0.4 mL/min Detector : RI Column temp. : 50 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Elution Volume of Saccharides
- Elution Volume of Saccharides (VG-50 4E)
- Separation of Anomer
- Comparison of Amino Column with Amide Column
- Comparison of Columns with Different Counter Ions
- Combination of Columns with Different Counter Ions
- Effects of Free Base Ratio of Amino Group on Plate Number (NH2P-50 4E)
- Effects of Acetonitrile Concentration on Elution Time (NH2P-50 4E)
- Effects of Acetonitrile Concentration on Elution Time (SZ5532)
- Effects of Acetonitrile Concentration on Plate Number (NH2P-50 4E)
- Durability Against Acidic Solvents (NH2P-50 4E)
- Durability Against Alkaline Solvents (NH2P-50 4E)
- Durability Against Eluent Composition Change (NH2P-50 4E)
- Effects of Sample Solvent Composition (NH2P-50 4E)
- Effects of Sample Injection Volume (NH2P-50 4E)
- Effects of Flow Rate (SC1011)
- Effects of Temperature (1) (SC1011)
- Effects of Temperature (2)
- Calibration Curves for Saccharides (NH2P-50 4E)
- Calibration Curves for Saccharides (SZ5532)
- Comparison of NH2P with Silica-based Amino Column (1)
- Comparison of NH2P with Silica-based Amino Column (2)
- Monosaccharides (1) (NH2P-50 4E)
- Monosaccharides (2) (NH2P-50 4E)
- Monosaccharides and Disaccharides (1) (SP0810)
- Monosaccharides and Disaccharides (2) (NH2P-50 4E)
- Monosaccharides and Disaccharides (3) (NH2P-50 4E)
- Monosaccharides and Disaccharides (4) (SZ5532)
- Monosaccharides and Disaccharides (5) (VG-50 4E)
- Mono-, Di- and Trisaccharides (1) (NH2P-50 4E)
- Saccharides in Agricultural Products (SC1011)
- Saccharides in Wood (1) (SP0810)
- Saccharides in Wood (2) (SP0810)
- Saccharides in Wood (3) (NH2P-50 4E)
- Palatinose in Food (SC1011)
- Worcester Sauce (KS-801)
- Yogurt: Sugar added (SP0810)
- Yogurt: Sugar added (NH2P-50 4E)
- Roast Sweet Potato (NH2P-50 4E)
- Roast Sweet Potato (SP0810)
- Soybean Flour (SP0810)
- Milk Coffee (NH2P-50 4E)
- Jelly (NH2P-50 4E)
- Saccharides in Bread (KS-801)
- Lactic acid Beverage and Fermented Milk (SH1011)
- Orange and Melon (SH1011)
- Orange Juice (SC1011)
- Chocolate Cake (NH2P-50 4E)
- Chocolate Cake (SZ5532)
- Maltose and Isomaltose (SZ5532)
- Saccharide Analysis Using Semi-micro Column (NH2P-50 2D)
- High Sensitive Analysis of Saccharides in powdered Milk (NH2P-50 4E)
- Glucose Derivatives (NH2P-50 4E)
- Extract of Wheat Rod (NH2P-50 4E)
- Effects of Organic Solvent in Eluent (NH2P-50 4E)
- Saccharides in Food (SC1011)
- Monosaccharides Composing Sugar Chain (SH1011)
- Maltose and Nigerose (NH2P-50 4E)
- Analysis of Ketohexose (SP0810)
- Cello-oligosaccharides and Ethanol (KS-802)
- Analysis of Rare Sugar (1) (SP0810)
- Analysis of Rare Sugar (2) (VG-50 4E)
- Galactinol (SC1011)
- Saccharides Separation in Presence of Na Salt (DC-613)
- Heptulose Separation (KS-801)
- Saccharides Analysis using Corona Charged Aerosol Detector (NH2P-50 4E)
- Comparison between NH2P-40 3E and its Conventional Type (NH2P-50 4E)
- Analysis of Saccharides and Furfurals (KS-801)
- Saccharides Analysis in Ionic Liquid (DC-613)
- Gulose (SP0810)
- Simultaneous Analysis of Lactose, Epilactose, and Lactulose (VG-50 4E)
- Analysis of Aldoses (VG-50 4E)
- Abnormal Behavior of Chromatogram of Saccharides Using a Pb Type Column for Ligand Exchange Chromatography
- Analysis of Fructose Glucose Syrup (High Fructose Syrup) According to Japanese Pharmaceutical Excipients Method (KS-801)
- Analysis of Ribose According to USP-NF Method (KS-801)
- Analysis of Dextrose According to USP-NF Method (SC1011)
- Analysis of Tagatose According to USP-NF Method (SC1011)
- Analysis of Maltose According to USP-NF Method (KS-801)
- Analysis of Trehalose According to USP-NF Method (KS-801)
- Analysis of Maltose Hydrate According to JP Method (KS-801)
- Analysis of Trehalose According to JP Method (KS-801)
- Analysis of Lactulose According to JP Method (SP0810)
- Analysis of Ethyl α-D-Glucoside in Sake (VG-50 4E)
- Analysis of Erythrose and Threose (SC1011)
- Analysis of 2-Deoxy-D-glucose (SC1011)
- Analysis of 2-Deoxy-D-glucose (VG-50 4E)
- Analysis of Dextrose and Fructose in Cranberry Juice (NH2P-50 4E)
