Although the analysis of glucosamine hydrochloride according to USP method should be used a column packed with L8 (aminopropylsilane chemically bonded to totally porous silica gel), silica-based amino columns are often highly durable. In this application, Asahipak NH2P-50 4D, a polymer based amino column, and a silica-based amino column from other manufacturer was compared for durability. In the case of the silica-based amino column, it began to deteriorate around 200 injections, and the column deteriorated sharply after 300 injections. On the other hand, NH2P-50 4D has no significant change in the theoretical plate numbers and tailing factor after 3,000 injections, proving its excellence in durability.
[Note] This analysis requires column equilibration.
For column equilibration: First introduce 60 mM Phosphoric acid aq. at 0.5 mL/min for 15 minutes, and then switch to the eluent at 0.9 mL/min over 3 hours.
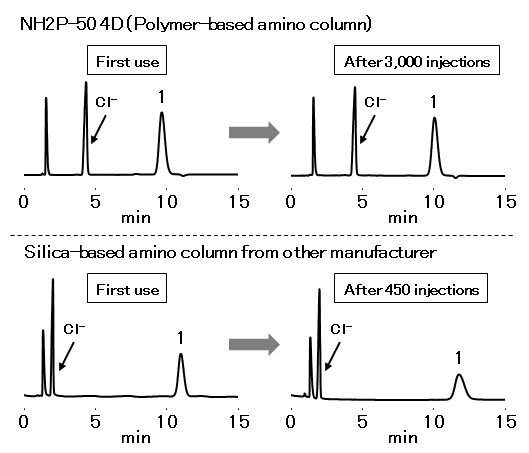
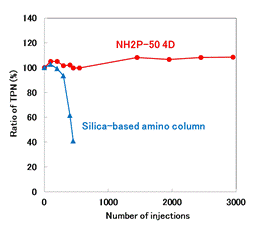
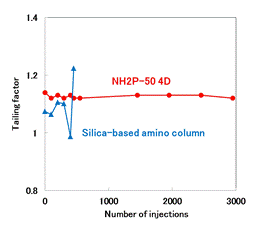
Sample : 3.8 mg/mL Glucosamine hydrochloride (in H2O/CH3CN=1/1), 10 μL
1. Glucosamine
Columns : Shodex Asahipak NH2P-50 4D (4.6 mm I.D. x 150 mm)
Silica-based amino column from other manufacturer (4.6 mm I.D. x 150 mm)
Eluent : *Buffer (pH7.5)/CH3CN=30/70 (NH2P-50 4D)
*Buffer (pH7.5)/CH3CN=25/75 (Silica-based amino column)
Flow rate : 0.9 mL/min (NH2P-50 4D)
1.5 mL/min (Silica-based amino column)
Detector : UV (195 nm)
Column temp. : 35 °C
*Buffer ; In a 1-L volumetric flask, dissolve 3.5 g K2HPO4 in water.
Add 0.25 mL Ammonium hydroxide (25 %), dilute with water to volume, and mix.
Adjusted with H3PO4 to a pH7.5
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Basic Drugs : Under Neutral Condition
- Basic Drugs : Effects of Eluent pH
- Basic Drugs : Effects of Ion Concentration
- Effects of pH of Eluent on Elution Volume and Plate Number (ODP-50 4D)
- Antifebriles (1) (DS-613)
- Antifebriles (2) (DM-614)
- Antipyretic Analgesics (ODP-50 4D)
- Diuretics (1) (ODP-50 4D)
- Diuretics (2) (DE-613)
- Anti Cancer Drugs (ODP-50 4D)
- Drugs (ODP-50 4D)
- Anti Inflammatory Drugs (1) (ODP-50 4E)
- Anti Inflammatory Drugs (2) (ODP-50 4E)
- Scopolamine and Atropine
- Tetracaine (ODP-50 4D)
- Procainamides (1) (DE-613)
- Procainamides (2) (DE-413)
- Procaine and Procaineamide (ODP-50 4D)
- Methylxantines (1) (DS-613)
- Methylxantines (2)
- Local Anesthetics (1) (ODP-50 4D)
- Local Anesthetics (2) (DE-413)
- Cinchona Alkaloids
- Antibiotics (1) (ODP-50 4D)
- Antibiotics (2) (DE-413)
- Sulfanilamides (DE-413)
- Barbiturates (ODP-50 4D)
- Effects of pH of Eluent on Sensitivity of Barbital
- Berberine in Japanese Goldthread (ODP-50 4D)
- Glycyrrhizin in Licorice (ODP-50 6D)
- Saikosaponins (ODP-50 6D)
- Soyasaponin in Soy Bean (DS-613)
- Curative Drugs to Eradicate H.Pyrori (JJ-50 2D)
- Enalapril Maleate (DS-413)
- Tetracycline Derivative Antibiotics (ODP-40 4D)
- Short Amines (ODP2 HP)
- Ginsenoside (Ginseng Saponin) (DS-413)
- Ginsenoside (Carrot Saponin) (NH2P-50 4E)
- Analysis of Macrolide Antibiotics and Derivatives (ODP-50 4D)
- Ribavirin (KS-801)
- Anti-diabetes Drugs (NH2P-50 4E)
- LC/MS/MS Analysis of Oral Anti-diabetes Drugs (VC-50 2D)
- Characterization of Polymeric Excipients by SEC-IR (SB-806M HQ)
- Simultaneous Analysis of Multi Symptom Cold Formula (ODP2 HP)
- LC/MS Analysis of Statins (ODP2 HP-2B)
- LC/MS Analysis of Metformin (NH2P-40 2D)
- Separation of Gingerol and Shogaol (C18M 4D)
- Bibliographic Information for Analysis of Conjugate Vaccine
- LC/MS/MS Analysis of Organic Sulfonic Acids (VT-50 2D)
- Analysis of Allantoin in a Commercial Disinfectant (VG-50 4E)
- LC/MS Analysis of Aminoglycoside Antibiotics (VC-50 2D)
- LC/MS/MS Analysis of Ribavirin (VC-50 2D)
- LC/MS/MS Analysis of Salacia Extract (1) (VC-50 2D)
- LC/MS/MS Analysis of Salacia Extract (2) (VT-50 2D)
- LC/MS Analysis of Streptomycins (VC-50 2D)
- LC/MS/MS Analysis of Citicoline (VT-50 2D)
- SEC Analysis of Liraglutide Formulation (KW-802.5)
- SEC Analysis of Antibody Drug Conjugate (LW-803)
- SEC Analysis of Saccharated Ferric Oxide Injection (SB-804 HQ + SB-802.5 HQ)
- Analysis of α-GPC in Supplements (NH2P-50 4E)
- SEC Analysis of Rituximab (LW-803)
- Analysis of Trypsin-Digested Rituximab (C18U 2D)
- PEG-Chymotrypsin (KW-802.5)
- SEC Analysis of PEG-Asparaginase (SB-806 HQ)
- LC/MS Analysis of Macrolide Antibiotics (C18U 2B)
- SEC Analysis of Filgrastim (KW-803)
- Analysis of Levocarnitine (NH2P-50 4E)
- Analysis of Inositol According to Japanese Pharmaceutical Excipients 2018 (KS-801)
- Analysis of Sodium Risedronate (IC I-524A)
- Analysis of Ibandronate Sodium (I-524A)
- Analysis of Zoledronic Acid (IC I-524A)
- Separation of Fosfomycin and its Thermal Decomposition Products (NH2P-50 4E)
- Analysis of Semaglutide Formulation (KW-802.5)
- Analysis of Ferric Carboxymaltose (SB-804 HQ + SB-802.5 HQ)
- Quantitative Analysis of Sucrose in Saccharated Iron Oxide Injection (VG-50 4E)
- Analysis of Carboplatin (NH2P-50 4E)
- Separation of Ceftazidime and Arginine (KW402.5-4F)
- Analysis of Sorbitol in Nicardipine Hydrochloride Injection (NH2P-50 4E)
- SEC Analysis of Etelcalcetide Hydrochloride (KW-802.5)
