A commercial rituximab drug (anti-CD20 monoclonal antibody) was analyzed using an aqueous SEC (GFC) column, PROTEIN LW-803. In addition to the mAb peak, there were oligomer (aggregate) and fragment peaks that were considered to be the decomposition product of mAb. Moreover, by using two columns in series, a fragment which have retention time very close to the monomer was detected. The LW-803 has an improved separation ability in the molecular weight range of IgG, thus it provides superior analytical capability for the quality control of antibody production to monitor not only the high-molecular weight impurities, aggregates, but also fragmentation.
*Between aggregates and monomer, use of one column generally provides a sufficient separation, however between fragment and monomer, use of two columns in series may provide a superior separation.
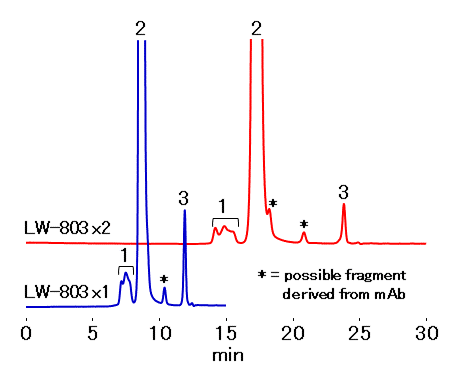
Sample : 20 µL
Lituximab formulation
(Injection solution)
1. Aggrigates
2. Monomer
3. Citrate
Column : Shodex PROTEIN LW-803 (8.0 mm I.D. x 300 mm) Eluent : 50 mM Sodium phosphate buffer (pH7.0) + 0.3 M NaCl Flow rate : 1.0 mL/min Detector : UV (280 nm) Column temp. : 25 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Basic Drugs : Under Neutral Condition
- Basic Drugs : Effects of Eluent pH
- Basic Drugs : Effects of Ion Concentration
- Effects of pH of Eluent on Elution Volume and Plate Number (ODP-50 4D)
- Antifebriles (1) (DS-613)
- Antifebriles (2) (DM-614)
- Antipyretic Analgesics (ODP-50 4D)
- Diuretics (1) (ODP-50 4D)
- Diuretics (2) (DE-613)
- Anti Cancer Drugs (ODP-50 4D)
- Drugs (ODP-50 4D)
- Anti Inflammatory Drugs (1) (ODP-50 4E)
- Anti Inflammatory Drugs (2) (ODP-50 4E)
- Scopolamine and Atropine
- Tetracaine (ODP-50 4D)
- Procainamides (1) (DE-613)
- Procainamides (2) (DE-413)
- Procaine and Procaineamide (ODP-50 4D)
- Methylxantines (1) (DS-613)
- Methylxantines (2)
- Local Anesthetics (1) (ODP-50 4D)
- Local Anesthetics (2) (DE-413)
- Cinchona Alkaloids
- Antibiotics (1) (ODP-50 4D)
- Antibiotics (2) (DE-413)
- Sulfanilamides (DE-413)
- Barbiturates (ODP-50 4D)
- Effects of pH of Eluent on Sensitivity of Barbital
- Berberine in Japanese Goldthread (ODP-50 4D)
- Glycyrrhizin in Licorice (ODP-50 6D)
- Saikosaponins (ODP-50 6D)
- Soyasaponin in Soy Bean (DS-613)
- Curative Drugs to Eradicate H.Pyrori (JJ-50 2D)
- Enalapril Maleate (DS-413)
- Tetracycline Derivative Antibiotics (ODP-40 4D)
- Short Amines (ODP2 HP)
- Ginsenoside (Ginseng Saponin) (DS-413)
- Ginsenoside (Carrot Saponin) (NH2P-50 4E)
- Analysis of Macrolide Antibiotics and Derivatives (ODP-50 4D)
- Ribavirin (KS-801)
- Anti-diabetes Drugs (NH2P-50 4E)
- LC/MS/MS Analysis of Oral Anti-diabetes Drugs (VC-50 2D)
- Characterization of Polymeric Excipients by SEC-IR (SB-806M HQ)
- Simultaneous Analysis of Multi Symptom Cold Formula (ODP2 HP)
- LC/MS Analysis of Statins (ODP2 HP-2B)
- LC/MS Analysis of Metformin (NH2P-40 2D)
- Separation of Gingerol and Shogaol (C18M 4D)
- Bibliographic Information for Analysis of Conjugate Vaccine
- LC/MS/MS Analysis of Organic Sulfonic Acids (VT-50 2D)
- Analysis of Allantoin in a Commercial Disinfectant (VG-50 4E)
- LC/MS Analysis of Aminoglycoside Antibiotics (VC-50 2D)
- LC/MS/MS Analysis of Ribavirin (VC-50 2D)
- LC/MS/MS Analysis of Salacia Extract (1) (VC-50 2D)
- LC/MS/MS Analysis of Salacia Extract (2) (VT-50 2D)
- Comparison of Analysis of Glucosamine Hydrochloride between Polymer-based Amino Columns and Silica-based Amino Columns
- LC/MS Analysis of Streptomycins (VC-50 2D)
- LC/MS/MS Analysis of Citicoline (VT-50 2D)
- SEC Analysis of Liraglutide Formulation (KW-802.5)
- SEC Analysis of Antibody Drug Conjugate (LW-803)
- SEC Analysis of Saccharated Ferric Oxide Injection (SB-804 HQ + SB-802.5 HQ)
- Analysis of α-GPC in Supplements (NH2P-50 4E)
- Analysis of Trypsin-Digested Rituximab (C18U 2D)
- PEG-Chymotrypsin (KW-802.5)
- SEC Analysis of PEG-Asparaginase (SB-806 HQ)
- LC/MS Analysis of Macrolide Antibiotics (C18U 2B)
- SEC Analysis of Filgrastim (KW-803)
- Analysis of Levocarnitine (NH2P-50 4E)
- Analysis of Inositol According to Japanese Pharmaceutical Excipients 2018 (KS-801)
- Analysis of Sodium Risedronate (IC I-524A)
- Analysis of Ibandronate Sodium (I-524A)
- Analysis of Zoledronic Acid (IC I-524A)
- Separation of Fosfomycin and its Thermal Decomposition Products (NH2P-50 4E)
- Analysis of Semaglutide Formulation (KW-802.5)
- Analysis of Ferric Carboxymaltose (SB-804 HQ + SB-802.5 HQ)
- Quantitative Analysis of Sucrose in Saccharated Iron Oxide Injection (VG-50 4E)
- Analysis of Carboplatin (NH2P-50 4E)
- Separation of Ceftazidime and Arginine (KW402.5-4F)
- Analysis of Sorbitol in Nicardipine Hydrochloride Injection (NH2P-50 4E)
- SEC Analysis of Etelcalcetide Hydrochloride (KW-802.5)
