According to the United States Pharmacopeia and the National Formulary (USP 36-NF 31*), the assay of xylitol and analysis of related impurities (other polyols) should be carried out with a column with L34 packing material and meets the requirements summerized in below table. The SUGAR SP0810 confirmed the requirements were met.
*The version at the time of the application acquisition.
- Note:
- This analysis is performed at high temperature (80 ℃). The following is the procedure at the beginning and end of the analysis.
- (At the beginning of analysis) Set the initial flow rate at 0.2 to 0.3 mL/min and start the system. If the column is to be heated during the analysis, keep the low flow rate until the column temperature reaches to the set temperature, and then gradually increase the flow rate to the desired.
- (At the end of analysis) Reduce the flow rate to 0.2 ~ 0.3 mL/min, allow the column temperature to drop to room temperature, and then stop the pump.
- *Please read the operation manual carefully to achieve the best and consistent column performance for a long time.
System suitability requirements Scroll
| Assay | Impurities analysis | |
|---|---|---|
| Relative retention time | Xylitol (1.0) Galactitol (1.10) |
L-Arabitol (0.76) Mannitol (0.81) Xylitol (1.0) Galactitol (1.12) Sorbitol (1.22) |
| Resolution | ≥ 2.0 (Xylitol/Galactitol) | ≥ 1.5 (Adjacent polyols) |
| Relative standard deviation (RSD) | ≤ 2% (Xylitol) | ≤ 5% (Galactitol) |
Sample: 25 µL
- 1.Xylitol 25 mg/mL
- 2.Galactitol 2.5 mg/mL
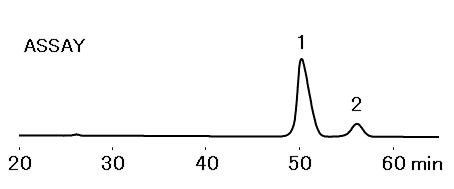
Sample: 25 µL
- 1.L-Arabinitol 0.5 mg/mL
- 2.Mannitol 0.5 mg/mL
- 3.Xylitol 100 mg/mL
- 4.Galactitol 0.5 mg/mL
- 5.Sorbitol 0.5 mg/mL
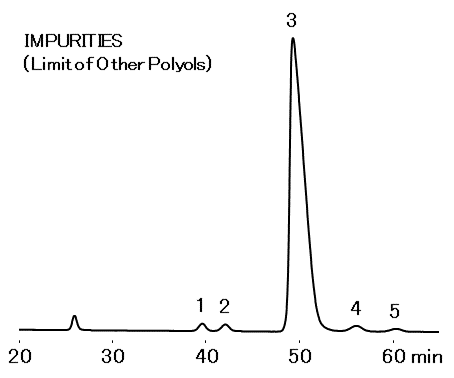
- Column
- :Shodex SUGAR SP0810 (8.0 mm I.D. x 300 mm)
- Eluent
- :H2O/CH3CN=80/20
- Flow rate
- :0.5 mL/min
- Detector
- :UV (192 nm)
- Column temp.
- :80 ℃
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Elution Volume of Saccharides and Sugar Alcohols (NH2P-50 4E)
- Sugar Alcohols (1) (SC1211)
- Sugar Alcohols (2) (NH2P-50)
- Sugar Alcohols (3)
- Saccharides and Sugar Alcohols (1) (SZ5532)
- Saccharides and Sugar Alcohols (2) (SP0810)
- Saccharides and Sugar Alcohols (3) (SP0810)
- Saccharides and Sugar Alcohols (4) (SC1011)
- Saccharides and Sugar Alcohols (5) (SZ5532)
- Saccharides and Sugar Alcohols (6) (NH2P-50)
- Saccharides and Amino Acids (1) (SC1011)
- Saccharides and Amino Acids (2) (SC1211)
- Saccharides and Amino Acids (3) (KS-801)
- Saccharides and Amino Sugars (2) (NH2P-50 4E)
- Saccharides and Amino Sugars (3) (SC1011)
- Xilitol in Tablet Candy
- Apple Juice (NH2P-50 4E)
- Apple Juice (SC1011)
- Sake (Japanese Rice Wine)
- Effects of Acetonitrile Concentration in Analysis of Sugar Alcohols
- Amino Sugars (SB-802.5 HQ)
- Soft Drink Containing Erythritol (NH2P-50 4E)
- Palatinit (SP0810, SC1011, SZ5532)
- Analysis of Pinitol (SP0810)
- Analysis of Meglumine (NN-814)
- LC/MS/MS Analysis of Meglumine (VC-50 2D)
- Analysis of Meglumine (YS-50)
- Separation of myo-Inositol and D-chiro-Inositol (SZ5532)
- Separation of Taurine and Inositol (NH2P-50 4E)
- Analysis of Hydrogenated Maltose Starch Syrup According to Japanese Pharmaceutical Excipients Method (KS-801)
- Analysis of Mannitol According to Pharmacopeia Method (SC1011-7F)
- Analysis of myo-Inositol According to EP Method (SC1011-7F)
- Analysis of Sorbitol According to USP-NF Method (SP0810 8C)
- Analysis of Maltitol According to USP-NF Method (SP0810 8C)
- Comparison of Sensitivity of NH2P Polymer-based Column and Silica-based Amino Column with ELSD
- Analysis of Erythrose and Erythritol (VG-50 4E)
