According to the United States Pharmacopeia and the National Formulary (USP 37-NF 32*), the European Pharmacopoeia (EP 9.0*), and the Japanese Pharmacopoeia (JP; 17th edition*), analysis of mannitol should meets following requirements. The SC1011-7F confirmed the requirements were met.
System suitability requirements:
Resolution of mannitol and sorbitol: ≥ 2.0
*The version at the time of the application acquisition.
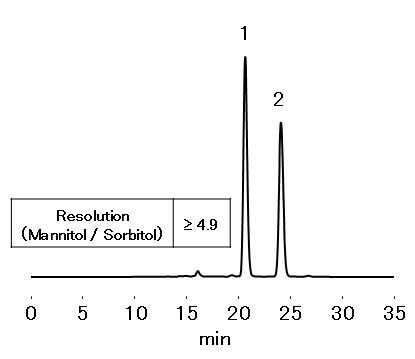
Sample : 25 mg/mL each, 20 µL
1. Mannitol
2. Sorbitol
Sample : 1 mg/mL each, 20 µL
3. Isomalt (= Palatinit)
4. Maltitol
Column : Shodex EP SC1011-7F (7.8 mm I.D. x 300 mm) Eluent : H2O Flow rate : 0.5 mL/min Detector : RI Column temp. : 85 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Elution Volume of Saccharides and Sugar Alcohols (NH2P-50 4E)
- Sugar Alcohols (1) (SC1211)
- Sugar Alcohols (2) (NH2P-50)
- Sugar Alcohols (3)
- Saccharides and Sugar Alcohols (1) (SZ5532)
- Saccharides and Sugar Alcohols (2) (SP0810)
- Saccharides and Sugar Alcohols (3) (SP0810)
- Saccharides and Sugar Alcohols (4) (SC1011)
- Saccharides and Sugar Alcohols (5) (SZ5532)
- Saccharides and Sugar Alcohols (6) (NH2P-50)
- Saccharides and Amino Acids (1) (SC1011)
- Saccharides and Amino Acids (2) (SC1211)
- Saccharides and Amino Acids (3) (KS-801)
- Saccharides and Amino Sugars (2) (NH2P-50 4E)
- Saccharides and Amino Sugars (3) (SC1011)
- Xilitol in Tablet Candy
- Apple Juice (NH2P-50 4E)
- Apple Juice (SC1011)
- Sake (Japanese Rice Wine)
- Effects of Acetonitrile Concentration in Analysis of Sugar Alcohols
- Amino Sugars (SB-802.5 HQ)
- Soft Drink Containing Erythritol (NH2P-50 4E)
- Palatinit (SP0810, SC1011, SZ5532)
- Analysis of Pinitol (SP0810)
- Analysis of Meglumine (NN-814)
- LC/MS/MS Analysis of Meglumine (VC-50 2D)
- Analysis of Meglumine (YS-50)
- Separation of myo-Inositol and D-chiro-Inositol (SZ5532)
- Separation of Taurine and Inositol (NH2P-50 4E)
- Analysis of Hydrogenated Maltose Starch Syrup According to Japanese Pharmaceutical Excipients Method (KS-801)
- Analysis of myo-Inositol According to EP Method (SC1011-7F)
- Analysis of Xylitol According to USP-NF Method (SP0810)
- Analysis of Sorbitol According to USP-NF Method (SP0810 8C)
- Analysis of Maltitol According to USP-NF Method (SP0810 8C)
- Comparison of Sensitivity of NH2P Polymer-based Column and Silica-based Amino Column with ELSD
- Analysis of Erythrose and Erythritol (VG-50 4E)
