The HPLC analysis of drugs in biological fluids usually requires the removal of the proteins present in the sample to avoid column contamination. This is not the case with the ODP2 HP reversed phase chromatography column. A high removal rate of sample proteins can be achieved thanks to the packing surface’s high polarity and small pore size. With ODP2 HP, proteins are naturally evacuated as waste product, resulting in shortened sample pre-treatment times. In this application, Risperidone was analyzed in 5-fold diluted urine injected directly into the column without further pretreatment.
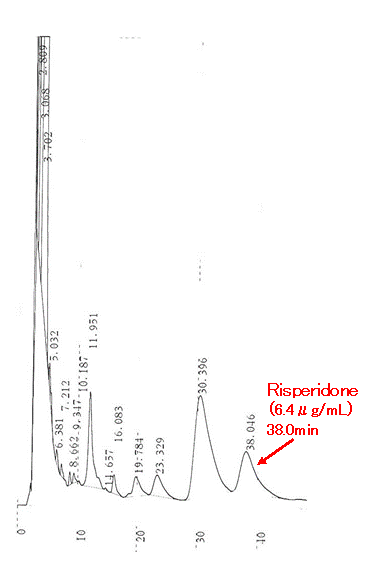
Sample : Human urine (5 fold diluted), 10 μL
Risperidone
Department of Clinical Sciences and Laboratory Medicine, Kansai Medical University.

Sample : Human urine (5 fold diluted), 10 μL
Risperidone
Column : Shodex ODP2 HP-4D (4.6 mm I.D. x 150 mm) Eluent : 0.1 % TFA:CH3CN=93:7 Flow rate : 0.6 mL/min Detector : UV (215 nm) Column temp. : 40 °CData courtesy of Katsuko Hara.MT Yutaka Komiyama.PhD,
Department of Clinical Sciences and Laboratory Medicine, Kansai Medical University.
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Indomethacin in Serum (ODP-50 6E)
- Procainamide in Serum (ODP-50 4D)
- LC/MS Analysis of Barbital in BSA (ODP2 HP-2B)
- LC/MS Analysis of Drugs in BSA (1) (ODP2 HP)
- LC/MS Analysis of Drugs in BSA (2) (ODP2 HP)
- LC/MS/MS Analysis of Tricyclic Antidepressants in BSA (ODP2 HP)
- LC/MS Analysis of Drugs in Control Serum (ODP2 HP)
- Reproducibility in LC/MS Analysis (ODP2 HP)
- Influence of Proteins in LC/MS Analysis (ODP2 HP-2B)
- Direct Analysis of Acetaminophen in Serum (ODP2 HP)
- Simultaneous Analysis of Multi Symptom Cold Formula in Serum (ODP2 HP)
- Analysis of Anticonvulsant in Serum (ODP2 HP)
- Hippuric Acid etc. in Urine (2)
