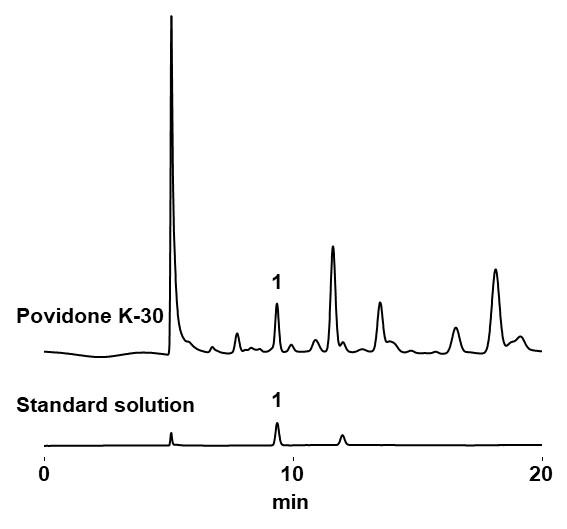
According to the Japanese Pharmacopeia (JP; 17th edition*), the United States Pharmacopeia and the National Formulary (USP43-NF38*), and the European Pharmacopoeia (EP 10.4*), analysis of formic acid in povidone should be carried out with a method that meets following requirements. The RSpak KC-811 confirmed the requirements were met. Using the same condition, we analyzed povidone K-30. The elution of formic acid was observed by comparing the elution position of the formic acid standard.
System suitability requirements
Theoretical plate number: ≥ 1,000
Symmetry factor: 0.5 – 1.5
Relative standard deviation (RSD) of six analyses: ≤ 2.0%
*The version at the time of the application acquisition.
Sample: 50 µL
Povidone K-30 20 mg/mL
Standard solution (Formic acid aq. 0.01 mg/mL)
- 1.Formic acid
- Column
- :Shodex RSpak KC-811 (8.0 mm I.D. x 300 mm)
- Eluent
- :Diluted HClO4 (70 %) (1 in 700)
- Flow rate
- :1.0 mL/min
- Detector
- :UV (210 nm)
- Column temp.
- :35 ℃
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Effects of Flow Rate (KC-811)
- Effects of Injection Volume (KC-811)
- Effects of Sample Load (KC-811)
- Effects of Column Temperature (KC-811)
- Effects of Concentration of Eluent on Elution Volume (KC-811)
- Effects of Column Temperature on Elution Volume (1) (KC-811)
- Effects of Column Temperature on Elution Volume (2) (KC-811)
- Effects of Acetonitrile in Eluent (1) (KC-811)
- Effects of Acetonitrile in Eluent (2) (KC-811)
- Effects of Reversed Phase Column on Elution Volume (KC-811 + DE-613)
- Elution Volume of Aromatic Organic Acids (NN-814)
- Elution Volume of Organic Acids (NN-814)
- Elution Volume of Organic Acids (DE-413)
- Comparison of Analysis of Organic Acids with 4 Kinds of Detectors (Standards) (KC-811)
- Comparison of Analysis of Organic Acids with 4 Kinds of Detectors (Soy Sauce) (KC-811)
- Organic Acid Standards (1) (KC-811)
- Organic Acid Standards (2) (KC-811)
- Organic Acid Standards (3) (KC-811)
- Organic Acid Standards (4) (DE-413)
- Organic Acid Standards (5) (DE-213)
- Citric Acid in Phosphoric Acid (DE-613 + KC-811)
- Analysis of Organic Acids in Beer (KC-811)
- Analysis of Organic Acids and Vitamin C in Fruit Juice (KC-811)
- Reverse Phase Analysis of Gluconic Acids (DE-613)
- Analysis of Organic Acids in Sake (Japanese Rice Wine) (KC-811)
- Analysis of Organic Acids in White Wine (KC-811)
- Analysis of Oxalic Acid Using Ion Exclusion Mode (KC-811)
- Organic Acid in Phosphoric Acid (DE-613 + KC-811)
- Analysis of Organic Acids in Soy Sauces (KC-811)
- Organic Acids in Vinegar (KC-811)
- Separation of Succinic Acid and Fumaric Acid (KC-811)
- Analysis of Organic Acids (Eluent Conteinig Cyclodextrin) (KC-811)
- Camphor and Benzoic Acid (NN-814)
- Di-carboxylic Acids (DE-413)
- Analysis of Organic Acids in Soybean Paste (KC-811)
- Analysis of Organic Acids in Soy Sauce For Noodles (KC-811)
- Organic Acids Analysis in Black-vinegar (KC-811)
- LC/MS Analysis of Organic Acids (DE-213)
- Simultaneous Analysis of Saccharides, Organic Acids and Amino Acids with LC/MS (VG-50 2D)
- Simultaneous Analysis of Hydrophilic Substances in Energy Drink with LC/MS (VG-50 2D)
- Organic Acids (KC-811)
- LC/MS Analysis of Pipecolic Acid (NH2P-40 2D)
- Elution Volume of Organic Acids (NI-424)
- Analysis of Related Compounds of Alanine and Aspartic Acid According to USP-NF Method (SH1011)
- Analysis of Acetic Acid in Vinegar Drinks (KC-811)
- Citric Acid Analysis in Functional Beverage (KC-811)
- Organic Acid Determination Test for Glycerin Fatty Acid Esters According to Japan’s Specifications and Standards for Food Additives (SH1011) (SH1011)
