Antibody drugs are produced from animal cells, and thus modification of proteins after their translation may result in variation of product’s uniformity. Consistent uniformity is important for maintaining the quality of biopharmaceuticals. Since uniformity variation can influence the functionalities of produced drugs, various quality assessments are required. One of them is analysis of charge variant (charge isomer). Charge variants are often observed for monoclonal antibodies, which are produced by deamidation of aspartyl residue or incomplete processing of H chain C-terminal lysine residue. Therefore, strict management of their profiles becomes important.
IEC SP-FT 4A, a strong cation exchange column packed with non-porous gel, was used for the analysis of commercial trastuzumab drug. A salt gradient method by increasing the NaCl concentration was used. SP-FT 4A provided higher separation efficiency in a shorter analysis time of charge variant than other company’s weak cation exchange column.
Sample: 5 μL
Trastuzumab formulation 5 mg/mL (in H2O)
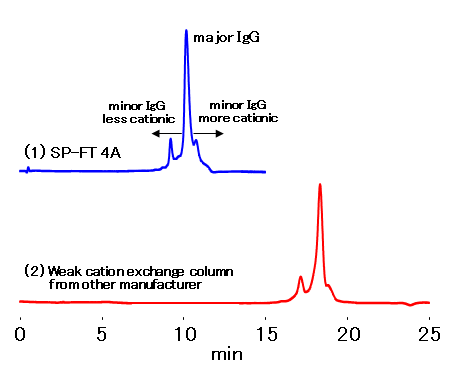
- Column
- :(1) Shodex IEC SP-FT 4A (4.6 mm I.D. x 10 mm ; 2.7 μm)
(2) Weak cation exchange column from other manufacturer (4.0 mm I.D. x 250 mm ; 10 μm) - Eluent
- :(A); 20 mM *MES buffer (pH7.0), (B); (A) + 0.5 M NaCl *MES : 2-(N-Morpholino)ethanesulfonic acid
Low pressure linear gradient;
(1) B % = 0 % to 10 % (10 min)
(2) B % = 0 % to 20 % (20 min) - Flow rate
- :(1) 0.5 mL/min
(2) 1.5 mL/min - Detector
- :UV (280 nm)
- Column temp.
- :40 ℃
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
