According to the “Japanese Pharmaceutical Excipients 2018”, quantification of purified stevia extract should be carried out with a column filled with aminopropyl silylated silica gel, while setting the flow rate to make the retention time of stevioside around 10 minutes and meeting following requirements.
The system suitability requirements:
The elution order to be rubusoside, dulcoside A, stevioside, rebaudioside C, and rebaudioside A
Resolution between stevioside and rebaudioside C: ≥ 1.5
Relative standard deviation (RSD)*: ≤ 1.5 %
*Injection of 20-µL stevioside standard (n=6)
The HILICpak VG-50 4E confirmed the requirements were met, and thus is suitable for the quantification of purified stevia extract. Compared to regular silica amino columns, VG-40 4E has two major advantages: 1. VG-50 4E reduces the eluent consumption to a half of a regular silica amino column of the same size uses, as it can be operated with less than a half flow-rate of silica amino column. 2. Since VG-50 4E is a polymer-based amino column, it has higher durability than silica-based amino column.
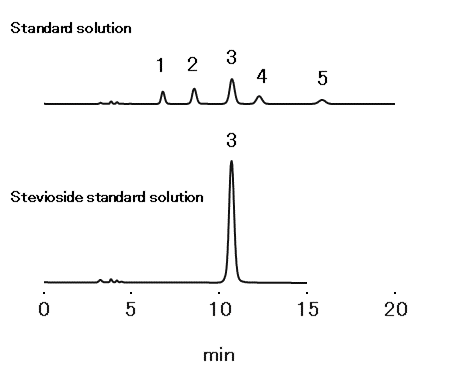
Standard solution
Sample: 20 µL
1. Rubusoside 0.003 %
2. Dulcoside A 0.003 %
3. Stevioside 0.01 %
4. Rebaudioside C 0.003 %
5. Rebaudioside A 0.005 %
Stevioside standard solution
Sample: 20 µL
3. Stevioside 0.05 %
Column : Shodex HILICpak VG-50 4E (4.6 mm I.D. x 250 mm) Eluent : H2O/CH3CN=20/80 Flow rate : 0.65 mL/min Detector : UV (213 nm) Column temp. : 35 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Acesulfame K (SC1011)
- Analysis of Acesulfame K and Sucralose (SC1011)
- Sucralose (SP0810)
- Stevioside and Rebaudioside A (NH2P-50 4E)
- LC/MS Analysis of Various Steviol Glycosides (NH2P-40 2D)
- Steviol Purity Test of Purified Stevia Extracts According to Japanese Pharmaceutical Excipients Method (C18M 4E)
- Analysis of Mogroside V in Momordica Grosvenori (NH2P-50 4E)
- LC/MS Analysis of Various Sweeteners (NH2P-40 2D)
- Simultaneous Analysis of Sweeteners and Amino Acids in Energy Drink (NH2P-40 2D)
- Simultaneous Analysis of Saccharides and Sweeteners in Yogurt Drink (NH2P-40 2D)
- Effects of Eluent pH on HILIC Mode (VG-50 2D)
- Analysis of Thaumatin (SP-825)
