Size Exclusion Chromatography (SEC) separates solutes in a sample according to
molecular size of solutes. Pores of packing materials are used for the separation.
When performing SEC, it is assumed that no interaction between solutes and packing
material exists. Solutes to be analyzed by SEC mode have different aspects such
as hydrophobic or hydrophillic, polar or non-polar and ionic or non-ionic, therefore,
the selection of the eluent suited to the solutes is very important to realize
pure SEC mode.
Features of SEC
1) No interaction between solutes and packing material is assumed.
2) To prevent the interaction, selection of appropriate eluent is essential.
3) The order of elution is the order of molecular size of solutes (from larger to smaller molecular size.)
4) The solutes whose molecular size is larger than the pore size of packing material cannot be separated each other. The upper limit of the molecular size which can be separeted is called as "exclusion limit". The exclusion limit is peculiar to column type.
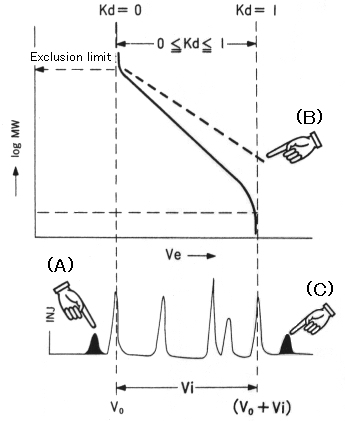
5) Elution volume which corresponds to exclusion limit is call as "void volume(Vo)", which is a very important characteristic of SEC column.
6) The total volume of pores is called as "inner volume(Vi)". The elution volumes of all solutes by pure SEC mode must exist between Vo and Vo + Vi.
Causes which prevent SEC mode
 When performing SEC mode analysis, the most important thing is how to realize pure SEC mode. For the analyses of polystyrene and saccharides, the optimal conditions to realize pure SEC mode are well-known. However, for some samples, it is difficult to find the optimal condition. And, sometimes, chromatograms which do not act on SEC mode appear or only poor reproducibility can be obtained.
When performing SEC mode analysis, the most important thing is how to realize pure SEC mode. For the analyses of polystyrene and saccharides, the optimal conditions to realize pure SEC mode are well-known. However, for some samples, it is difficult to find the optimal condition. And, sometimes, chromatograms which do not act on SEC mode appear or only poor reproducibility can be obtained.
When pure SEC mode is performed, the calibration curve exist in the range of Vo and Vo + Vi as shown by the solid line in the right figure. However, when pure SEC mode is not performed, the calibration curve shown by the broken line can be obtained.
In case (A), some solute elute before Vo. The possible causes of this too fast elution are as follows:
* When the sample is ionic and the eluent is not an appropriate one, this phenomenon may happen. Please refer to SEC Analysis of Ionic Sample. Even when the elution volumes are in the range of Vo and Vo + Vi, this may be the cause of poor repuroducibility.
* When the aggregation of sample is taken place, this phenomenon may happen.
* When the ion-exclusion with packing material is taken place, this phenomenon may happen.
In case (B) and (C), solute must be adsorbed by the packing material. Such adsorption is mainly caused by hydrophobic or phdrophillic interaction and sometimes by hydrogen bond or ionic bond. The way t prevent such adsorption is as follows:
* To prevent hydrophobic or phdrophillic interaction, an eluent which has higher solubility to the solute and/or higher affinity to the packing material should be used.
* For some ionic polymers, addition of LiCl or LiBr increases the solubility and
may solve the problem.
* When the sample is a polymer which has weak ionic group such as -COO-,
the affinity of the polymer can be largely affected by the degree of dissociation.
In such case, the degree of dissociation should be stabilized by using some buffer.
Applications
- Hydrophobic Polymers: THF Eluent
- Hydrophobic Polymers: Chloroform Eluent
- Hydrophobic Polymers: DMF Eluent
- Hydrophobic Polymers: HFIP Eluent
- Hydrophobic Polymers: Other Eluents
- Additives in Hydrophobic Polymers
- Hydrophobic Oligomers
- Hydrophilic Polymers
- Cationic Polymers
- Solvent-peak Separation Columns
Applications (Related Information)
- Guidelines for Shodex Column Selection
- Sample Concentration for SEC Analysis
- Effects of Sample Load (Pullulan)
- Selection of Calibration Curves
- Selection of Calibration Standards
- Connection of Different Pore-size Columns
- Comparison of Calibration Curves: Linear Type and Conventional Type
- Comparison of Separation: Linear Type and Conventional Type (1)
- Comparison of Separation: Linear Type and Conventional Type (2)
- Solubility of Solvents
- Physical Chemistry Properties of Solvents
- Polarities of Solvents
