According to the United States Pharmacopeia and the National Formulary (USP 40-NF 35*), analysis of ascorbic acid should be carried out with a column with L39 packing material and meets following requirements. The RSpak DM-614 confirmed the requirements were met.
System suitability requirements:
Tailing factor: ≤ 1,6
Theoretical plate number: ≥ 3,500
Relative standard deviation (RSD): ≤ 1.5%
*The version at the time of the application acquisition.
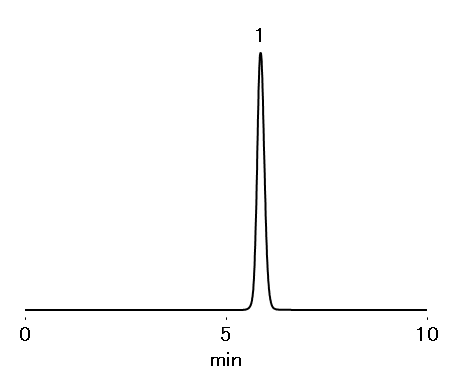
Sample : 4 μL
1. Ascorbic acid 0.05 %
Column : Shodex RSpak DM-614 (6.0 mm I.D. x 150 mm) Eluent : 0.055 M Na2HPO4 + 0.045 M KH2PO4 aq. (adjusted pH 2.5 with H3PO4) Flow rate : 0.6 mL/min Detector : UV (245 nm) Column temp. : 30 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Water-Soluble Vitamins (1) (DE-413)
- Water-Soluble Vitamins (4) (DM-614)
- Water-Soluble Vitamins (5) (NH2P-50 4E)
- Simultaneous Analysis of Water-Soluble Vitamins (DE-413)
- LC/MS Analysis of Water-soluble Vitamins (DE-213)
- Vitamin B6 (1) (ODP-50 6D)
- Vitamin B12 (SB-802.5 HQ)
- Vitamin C (1) (KC-811)
- Vitamin C (3) (NH2P-50 4E)
- Soft Drink Containing Vitamin C (NH2P-50 4E)
- LC/MS Analysis of Pantothenic Acid and Vitamin C (VT-50 2D)
- Separation of Ascorbic Acid and Isoascorbic Acid (DE-413)
- Carnitine (NN-814)
- LC/MS Analysis of Carnitine and Trimethyl Glycine (NH2P-40 2D)
- Food Containing Vitamins (DE-413)
- Nutrient Beverage (DE-413)
- Separation of Water-Soluble Vitamin and Sulfite (KC-811)
- Vitamin D (ODP-50 6E)
- Margarine (KF-801)
- Fat-Soluble Vitamins (1) (ODP-50 4E)
- Fat-Soluble Vitamins (3) (KF-801)
- Carotene Analysis in Carrot Juice (C18U 2D)
