Calcium acetate is a food additive often used in European and US countries as preservatives and pH adjusting agents, as well as to enhance the nutritional value of the food products.
According to the United States Pharmacopeia and the National Formulary (USP 43-NF 38*), analysis of calcium acetate capsules should be carried out with a column filled with L76 packing material and meets following requirements. The IC YK-421 confirmed the requirements were met.
System suitability requirements:
Theoretical plate number of calcium ion: ≥ 1,000
Relative standard deviation (RSD): ≤ 2.0%
*The version at the time of the application acquisition.
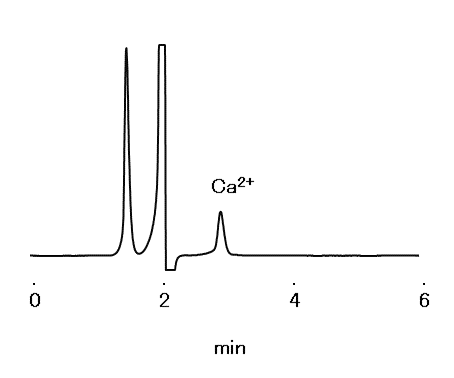
Sample : 0.08 mg/mL Calcium acetate in water, 10 µL
Column : IC YK-421 (4.6 mm I.D. x 125 mm) Eluent : 0.75 mM Dipicolinic Acid and 1.7 mM Nitric Acid in water/Acetone=900/100 Flow rate : 0.9 mL/min Detector : Non-suppressed conductivity Column temp. : 35 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Analysis of Erythropoietin According to EP Method (LW-803)
- Analysis of Somatropin According to EP Method (LW-803)
- Analysis of Teriparatide According to EP Method (KW-802.5)
- Analysis of Ethylcellulose Dispersion Type B According to USP-NF Method (KF-802)
- Analysis of Azithromycin According to USP-NF Method (ODP-50 4E)
- Analysis of Azithromycin Injection According to USP-NF Method (ODP-50 4D)
- Analysis of Ascorbic Acid Injection According to USP-NF Method (DM-614)
- Analysis of Related Compounds of Alanine and Aspartic Acid According to USP-NF Method (SH1011)
- Analysis of High Molecular Weight Protein According to USP-NF GENERAL CHAPTERS Physicochemical Analytical Procedures for Insulins (KW-802.5)
- Analysis of Exenatide According to USP-NF Method (KW-802.5)
- Analysis of Epoetin According to USP-NF Method (LW-803)
- Analysis of Oligomers in Omega-3 Free Fatty Acids According to USP-NF Method (KF-802.5 + KF-802 + KF801)
- Analysis of Xylitol According to USP-NF Method (SP0810)
- Analysis of Guar Gum According to USP-NF Method (SP0810)
- Analysis of Contaminants Sucrose and Sorbitol in Cranberry Juice According to USP-NF Method (SC1011-7F)
- Analysis of Zanamivir According to USP-NF Method (NH2P-50 4E)
- Quantification of N-Methylpyrrolidine in Cefepime for Injection According to USP-NF Method (YK-421)
- Analysis of Sorbitol According to USP-NF Method (SP0810 8C)
- Analysis of Tagatose According to USP-NF Method (SC1011)
- Analysis of Dextrose According to USP-NF Method (SC1011)
- Analysis of Trehalose According to USP-NF Method (KS-801)
- Analysis of Pamidronate Disodium According to USP-NF Method (IC I-524A)
- Analysis of Betadex Sulfobutyl Ether Sodium According to USP-NF Method (SB-803 HQ)
- Measurement of Apparent Weight-Average Molecular Weight and Polydispersity of Polyethylene Glycol 3350 According to USP-NF Method (SB-803 HQ)
- Assay of Polyethylene Glycol 3350 According to USP-NF Method (SB-802.5 HQ)
- Assay of Polyethylene Glycol 3350 for Oral Solution According to USP-NF Method (SB-802.5 HQ)
- Impurity Analysis of Voriconazole According to USP-NF Method (CDBS-453)
- Analysis of Maltitol According to USP-NF Method (SP0810 8C)
- Analysis of Maltose According to USP-NF Method (KS-801)
- Analysis of Propylene Glycol Monocaprylate According to USP-NF Method (KF-801)
- Impurity Analysis of Lamivudine According to USP-NF Method (CDBS-453)
- Analysis of Ribose According to USP-NF Method (KS-801)
- Analysis of Zinc Sulfate Compounded Injection According to USP-NF Method (YK-421)
- Analysis of Ibandronate Sodium Hydrate Proposed in USP-NF Pharmacopeial Forum (I-524A)
- Analysis of Sucrose Proposed in USP-NF Pharmacopeial Forum (SC1011-7F)
- Analysis of Urea, an Impurity in Fluorouracil Injection, Proposed in USP-NF Pharmacopeial Forum (SB-802.5 HQ)
- Analysis of Mannitol Injection Proposed in USP-NF Pharmacopeial Forum (SP0810)
- Analysis of Potassium Bicarbonate and Potassium Chloride Effervescent Tablets for Oral Solution According to USP-NF Method (YK-421)
- Analysis of Potassium and Sodium Bicarbonates and Citric Acid Effervescent Tablets for Oral Solution According to USP-NF Method (YK-421)
- Analysis of Glyceryl Stearate According to USP-NF Method (KF-802)
- Analysis of Glyceryl Distearate According to USP-NF Method (KF-802)
- Analysis of Glyceryl Tristearate According to USP-NF Method (KF-802)
- Molecular Weight Measurements of Low Molecular Weight Heparins According to USP-NF GENERAL CHAPTERS <209> (KW-803 + KW-802.5)
- Analysis of Azithromycin According to JP Method (ODP-50 4E)
- Quantification of Alendronate Sodium Hydrate According to JP Method (DS-413)
- Quantification of Alendronate Sodium Hydrate and Its Related Compound According to JP Method (DS-413)
- Quantification of Alendronate Sodium Injection According to JP Method (DS-613)
- Quantification of Alendronate Sodium Tablets According to JP Method (DS-613)
- Analysis of Insulin Glargine According to JP Method (KW-802.5)
- Analysis of Trehalose According to JP Method (KS-801)
- Analysis of Human Insulin According to JP Method (KW-802.5)
- Analysis of Voglibose According to JP Method (NH2P-50 4E)
- Analysis of Maltose Hydrate According to JP Method (KS-801)
- Analysis of Miglitol According to JP Method (NH2P-50 4E)
- Analysis of Lactulose According to JP Method (SP0810)
- Analysis of Purified Sodium Hyaluronate Ophthalmic Solution According to JP Method (SB-802.5 HQ)
- Analysis of High Molecular Impurities in Insulin Aspart According to Pharmacopeia Method (KW-802.5)
- Analysis of Formic Acid in Povidone According to Pharmacopeia Method (KC-811)
- Analysis of Formoterol Fumarate Dihydrate According to Pharmacopeia Method (ODP-50 4D)
- Analysis of Mannitol According to Pharmacopeia Method (SC1011-7F)
