According to the Japanese Pharmacopeia (JP; 17th edition*), analysis of purified sodium hyaluronate ophthalmic solution should meets following requirements. The OHpak SB-802.5 HQ confirmed the requirements were met.
System suitability requirements:
Resolution of Hyaluronic acid /ε-Aminocaproic acid: ≥ 5
Relative standard deviation (RSD) of six analyses: ≤ 2.0%
*The version at the time of the application acquisition.
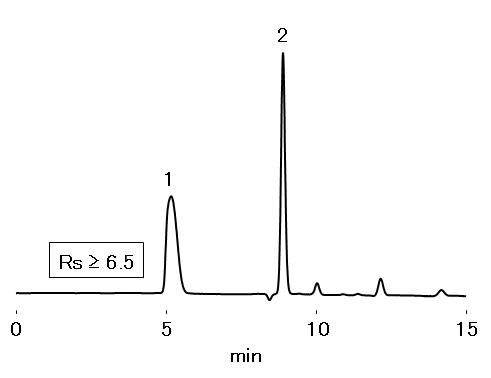
Sample : 20 μL
System suitability solution (prepared according to JP method)
1. Hyaluronic acid
2. ε-Aminocaproic acid
Column : Shodex OHpak SB-802.5 HQ (8.0 mm I.D. x 300 mm) Eluent : 0.1 M Na2SO4 aq. Flow rate : 1.0 mL/min Detector : UV (210 nm) Column temp. : 40 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Analysis of Erythropoietin According to EP Method (LW-803)
- Analysis of Somatropin According to EP Method (LW-803)
- Analysis of Teriparatide According to EP Method (KW-802.5)
- Analysis of Ethylcellulose Dispersion Type B According to USP-NF Method (KF-802)
- Analysis of Azithromycin According to USP-NF Method (ODP-50 4E)
- Analysis of Azithromycin Injection According to USP-NF Method (ODP-50 4D)
- Analysis of Ascorbic Acid Injection According to USP-NF Method (DM-614)
- Analysis of Related Compounds of Alanine and Aspartic Acid According to USP-NF Method (SH1011)
- Analysis of High Molecular Weight Protein According to USP-NF GENERAL CHAPTERS Physicochemical Analytical Procedures for Insulins (KW-802.5)
- Analysis of Exenatide According to USP-NF Method (KW-802.5)
- Analysis of Epoetin According to USP-NF Method (LW-803)
- Analysis of Oligomers in Omega-3 Free Fatty Acids According to USP-NF Method (KF-802.5 + KF-802 + KF801)
- Analysis of Xylitol According to USP-NF Method (SP0810)
- Analysis of Guar Gum According to USP-NF Method (SP0810)
- Analysis of Contaminants Sucrose and Sorbitol in Cranberry Juice According to USP-NF Method (SC1011-7F)
- Analysis of Zanamivir According to USP-NF Method (NH2P-50 4E)
- Quantification of N-Methylpyrrolidine in Cefepime for Injection According to USP-NF Method (YK-421)
- Analysis of Sorbitol According to USP-NF Method (SP0810 8C)
- Analysis of Tagatose According to USP-NF Method (SC1011)
- Analysis of Dextrose According to USP-NF Method (SC1011)
- Analysis of Trehalose According to USP-NF Method (KS-801)
- Analysis of Pamidronate Disodium According to USP-NF Method (IC I-524A)
- Analysis of Betadex Sulfobutyl Ether Sodium According to USP-NF Method (SB-803 HQ)
- Measurement of Apparent Weight-Average Molecular Weight and Polydispersity of Polyethylene Glycol 3350 According to USP-NF Method (SB-803 HQ)
- Assay of Polyethylene Glycol 3350 According to USP-NF Method (SB-802.5 HQ)
- Assay of Polyethylene Glycol 3350 for Oral Solution According to USP-NF Method (SB-802.5 HQ)
- Impurity Analysis of Voriconazole According to USP-NF Method (CDBS-453)
- Analysis of Maltitol According to USP-NF Method (SP0810 8C)
- Analysis of Maltose According to USP-NF Method (KS-801)
- Analysis of Propylene Glycol Monocaprylate According to USP-NF Method (KF-801)
- Impurity Analysis of Lamivudine According to USP-NF Method (CDBS-453)
- Analysis of Ribose According to USP-NF Method (KS-801)
- Analysis of Calcium Acetate Capsules According to USP-NF Method (YK-421)
- Analysis of Zinc Sulfate Compounded Injection According to USP-NF Method (YK-421)
- Analysis of Ibandronate Sodium Hydrate Proposed in USP-NF Pharmacopeial Forum (I-524A)
- Analysis of Sucrose Proposed in USP-NF Pharmacopeial Forum (SC1011-7F)
- Analysis of Urea, an Impurity in Fluorouracil Injection, Proposed in USP-NF Pharmacopeial Forum (SB-802.5 HQ)
- Analysis of Mannitol Injection Proposed in USP-NF Pharmacopeial Forum (SP0810)
- Analysis of Potassium Bicarbonate and Potassium Chloride Effervescent Tablets for Oral Solution According to USP-NF Method (YK-421)
- Analysis of Potassium and Sodium Bicarbonates and Citric Acid Effervescent Tablets for Oral Solution According to USP-NF Method (YK-421)
- Analysis of Glyceryl Stearate According to USP-NF Method (KF-802)
- Analysis of Glyceryl Distearate According to USP-NF Method (KF-802)
- Analysis of Glyceryl Tristearate According to USP-NF Method (KF-802)
- Molecular Weight Measurements of Low Molecular Weight Heparins According to USP-NF GENERAL CHAPTERS <209> (KW-803 + KW-802.5)
- Analysis of Azithromycin According to JP Method (ODP-50 4E)
- Quantification of Alendronate Sodium Hydrate According to JP Method (DS-413)
- Quantification of Alendronate Sodium Hydrate and Its Related Compound According to JP Method (DS-413)
- Quantification of Alendronate Sodium Injection According to JP Method (DS-613)
- Quantification of Alendronate Sodium Tablets According to JP Method (DS-613)
- Analysis of Insulin Glargine According to JP Method (KW-802.5)
- Analysis of Trehalose According to JP Method (KS-801)
- Analysis of Human Insulin According to JP Method (KW-802.5)
- Analysis of Voglibose According to JP Method (NH2P-50 4E)
- Analysis of Maltose Hydrate According to JP Method (KS-801)
- Analysis of Miglitol According to JP Method (NH2P-50 4E)
- Analysis of Lactulose According to JP Method (SP0810)
- Analysis of High Molecular Impurities in Insulin Aspart According to Pharmacopeia Method (KW-802.5)
- Analysis of Formic Acid in Povidone According to Pharmacopeia Method (KC-811)
- Analysis of Formoterol Fumarate Dihydrate According to Pharmacopeia Method (ODP-50 4D)
- Analysis of Mannitol According to Pharmacopeia Method (SC1011-7F)
