According to the United States Pharmacopeia and the National Formulary Pharmacopeial Forum (PF 44(6)*), enantiomeric purity of epinephrine injection should be carried out with a column filled with L45 packing material and meets following requirements. The use of ORpak CDBS-453 confirmed the requirements were met.
System suitability requirements:
Resolution of (R)-epinephrine and (S)–epinephrine (in system suitability solution): ≥ 1.5
Relative standard deviation (RSD) of the peak area : ≤ 2.0%
The signal / noise ratio (in the sensitivity solution): ≥ 10
*The version at the time of the application acquisition.
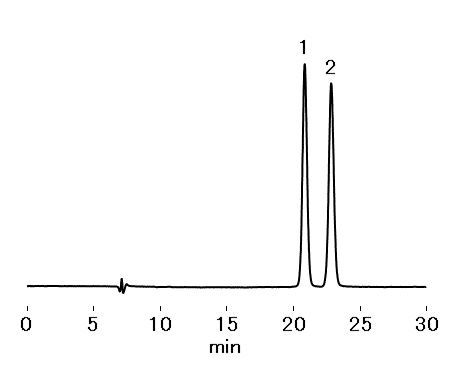
Sample : 10 μL
System suitability solution (Epinephrine hydrochloride 20 μg/mL (in *Solution A))
1. (R)-Epinephrine
2. (S)-Epinephrine
Column : Shodex ORpak CDBS-453 (4.6 mm I.D. x 150 mm)
Eluent : *Solution A/CH3CN=99/1
Flow rate : 0.3 mL/min
Detector : UV (280 nm)
Column temp. : 25 °C
*: 0.75 g/L Ammonium acetate aqueous solution adjusted to pH 4.0
with Glacial acetic acid
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
