According to Japanese Pharmaceutical Excipients 2018 method, steviol purity test of purified stevia extracts should be used the HPLC column packed with octadecylsilylated silica gel. The system suitability requires to satisfy resolution between steviol and the internal standard 4-tert-butyl benzoic acid, to be ≥ 6.0. Also, the relative standard deviation of steviol to be ≤ 1.5 % for the six repeated analysis. It was confirmed that Silica C18M 4E satisfies all required conditions.
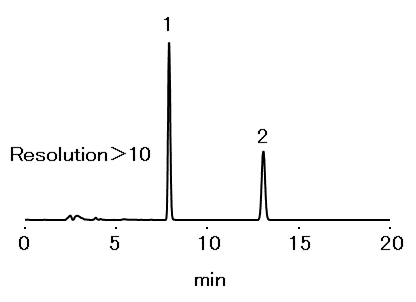
Sample : 20 μL
1. 4-tert-butyl benzoic acid 0.01 mg/mL
2. Steviol 0.05 mg/mL
Column : Shodex Silica C18M 4E (4.6 mm I.D. x 250 mm) Eluent : 0.085 % H3PO4/CH3CN=9/11 Flow rate : 1.0 mL/min Detector : UV (213 nm) Column temp. : 40 °C
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Acesulfame K (SC1011)
- Analysis of Acesulfame K and Sucralose (SC1011)
- Sucralose (SP0810)
- Stevioside and Rebaudioside A (NH2P-50 4E)
- LC/MS Analysis of Various Steviol Glycosides (NH2P-40 2D)
- Quantification of Purified Stevia Extract (VG-50 4E)
- Analysis of Mogroside V in Momordica Grosvenori (NH2P-50 4E)
- LC/MS Analysis of Various Sweeteners (NH2P-40 2D)
- Simultaneous Analysis of Sweeteners and Amino Acids in Energy Drink (NH2P-40 2D)
- Simultaneous Analysis of Saccharides and Sweeteners in Yogurt Drink (NH2P-40 2D)
- Effects of Eluent pH on HILIC Mode (VG-50 2D)
- Analysis of Thaumatin (SP-825)
