Guar gum is known to suppress blood sugar level raise and reduce cholesterol.
In the United States Pharmacopeia and the National Formulary (USP 39-NF 34*), the ratio of constituting mannose and galactose is defined, and quantification of guar gum should be carried out with a column with L22 packing material and meets following requirements. The SUGAR SP0810 confirmed the requirements were met.
*The version at the time of the application acquisition.
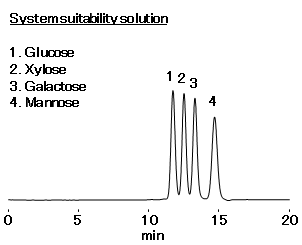
| Resolution in the system suitability solution | between Dextrose (= Glucose) and Xylose: ≥ 0.9 between Xylose and Galactose: ≥ 1.0 between Galactose and Mannose: ≥ 1.5 |
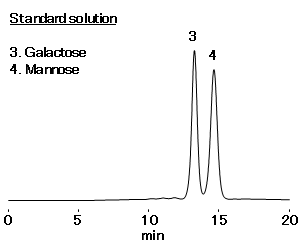
| Tailing factor in the standard solution | Galactose and Mannose peaks: 0.8 - 1.8 |
| Relative standard deviation (%RSD) in the standard solution | Galactose and Mannose peaks: ≥ 2.0 % (6 measurements) |
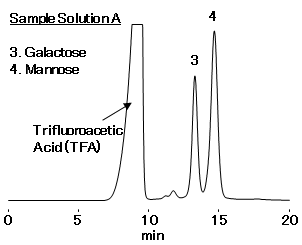
Sample Solution A : Guar gum dissolved in TFA aq. Mannose and glucose are produced as a result of hydrolysis of galactomannan by acids. The content of galactomannan and ratio of constituting mannose and galactose from the quantitative values of mannose and galactose in the portion of guar gum taken were calculated.
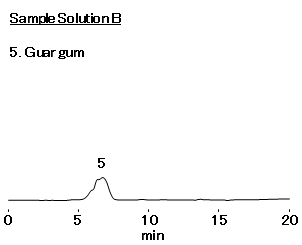
Sample Solution B : Guar gum dissolved in the eluent. No presence of galactose and mannose peaks are observed in the chromatogram of Standard solution B.
Sample: 10 μL
- System suitability solution:Glucose, Xylose, Galactose, Mannose 5 mg/mL each (in H2O)
- Standard solution:Galactose, Mannose 10 mg/mL each (in H2O)
- Sample Solution A:Guar gum 25 mg/mL (prepared according to USP method)
- Sample Solution B:Guar gum 5 mg/mL (in eluent)
- Column
- :Shodex SUGAR SP0810 (8.0 mm I.D. x 300 mm)
- Eluent
- : H2O
- Flow rate
- :0.75 mL/min
- Detector
- :RI
- Column temp.
- :80 ℃
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Heparin (1) (SB-804 HQ)
- Heparin (2) (SB-806M HQ)
- Heparin (3) (KW-804)
- Absolute Molecular Weight Determination of Heparin (LB-806M)
- SEC Analysis of Low Molecular Weight Heparin (1) (Dalteparin Sodium) (KW-803 + KW-802.5)
- SEC Analysis of Low Molecular Weight Heparin (2) (Parnaparin Sodium) (KW-803 + KW-802.5)
- Analysis of Enoxaparin Sodium (KW-803 + KW-802.5)
- Corn Starch (1) (Aqueous Eluent) (SB-806M HQ)
- Corn Starch (2) (DMSO/DMF Eluent) (SB-806 HQ)
- Corn Starch (3) (DMSO/DMF Eluent) (KD-806M)
- Hyaluronic Acid (1) (SB-805 HQ)
- Hyaluronic Acid (2) (SB-806M HQ)
- Hyaluronic Acid (4) (SB-807 HQ)
- Chondroitin Sulfate (SB-806M HQ)
- Chondroitin Sulfate A (KS-804)
- Chondroitin Sulfate (KS-804 + KS-801)
- Chitosan (SB-806M HQ)
- Pullulan and Oligosaccharides (KS-804)
- Pullulan, Saccharides and Ethanol (KS-802)
- Amylose (KS-802)
- Hydrolyzed Starch (1) (KS-802)
- Inulin (2) (SB-806M HQ)
- Mucin (SB-806M HQ)
- Pectin (SB-806M HQ)
- Glycogen (2) (SB-806M HQ)
- Sodium Alginate (SB-806M HQ)
- Absolute Molecular Weight Determination of Sodium Alginate (LB-806M)
- Carrageenan (SB-806M HQ)
- Keratan sulfate (SB-806M HQ)
- Carboxymethylcellulose (3) (SB-806M HQ)
- Carboxymethylcellulose (4) (SB-806M HQ)
- Hydroxyethyl Cellulose (3) (SB-806M HQ)
- Hydroxyethyl Cellulose (4) (SB-806M HQ)
- Potato Starch (DMSO/DMF Eluent: KD-806M)
- Analysis of Cyanobacterial Glycogen Using SEC Column (SB-806M HQ)
- Pullulan Standards (8) (LF-804)
- Xylan (LF-804)
- Cellulose (KD-806M)
- Pullulan Standards (1) (SB-804 HQ)
- Pullulan Standards (2) (GF-310 HQ)
- Pullulan Standards (4) (Effects of Flow Rate) (SB-2002.5)
- Pullulan Standards (5) (Effects of Temperature) (SB-2002.5)
- Dextran (1) (SB-805 HQ)
- Dextran (2) (DMSO Eluent) (KF-806M)
- Dextran (3) (SB-806M HQ)
- Sodium Dextran Sulfate (SB-806M HQ)
- Methylcellulose (SB-806M HQ)
- Hydroxypropyl Cellulose (SB-806M HQ)
- Hydroxypropyl Methylcellulose (SB-806M HQ)
- Arabic Gum (SB-806M HQ)
- Guar Gum (SB-806M HQ)
- Fucoidan (SB-806M HQ)
- Laminarin (SB-806M HQ)
- Analysis of Indigestible Dextrin in Beverage (LB-802.5)
- Analysis of Indigestible Dextrin Powder (LB-802.5)
Applications (Related Information)
- Calibration Curves for KS-800 Series (Aqueous Eluent: Pullulan)
- Calibration Curves for KS-800 Series (Column Combination)
- Calibration Curves for SB-800 HQ Series (Aqueous Eluent: Pullulan)
- Calibration Curves for SB-806M HQ (Aqueous Eluent: Pullulan, PEG and PEO)
- Calibration Curve for SB-807 HQ (Aqueous Eluent: Pullulan)
- Calibration Curves for GF-HQ Series (Aqueous Eluent: Pullulan)
- Calibration Curves for GS-HQ Series (Aqueous Eluent: Pullulan)
- Calibration Curve for LF-804 (DMSO/DMF Eluent: Pullulan)
