Saccharides exhibits an energy-stable chair conformation in 5-membered ring (furanose) or 6-membered ring (pyranose)
forms. Since hydroxyl group on each carbon can take either equatorial or axial position, even two saccharides having the
same molecular structures may have different three dimensional configurations. Ligand exchange mode separates
saccharides using this configuration difference of the complex formed between saccharides' hydroxyl groups and metal
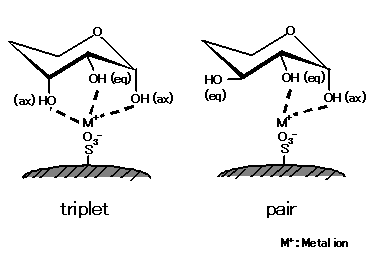
ions. As shown in the left figure, saccharides having a larger number of ax-eq-ax configuration (triplet) units form stronger
complexes with metal ions. Meanwhile, as right figure shows, saccharides lacking such a triplet structure form complexes
with ax-eq (pair) hydroxyl group. As the unit number of this pair structure increases, saccharides become more potent
forming complexes with metal ions. The complex formation capacity also differs depending on modified metal ions.
General Information on Packed Columns
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
