Antibody drugs have been spotlighted as therapeutic agents which have a high specificity to the target molecule with few side effects. It is known that dimer and/or larger aggregates may be produced during the manufacturing process or storage of antibody drugs. Since it is possible that these aggregates may become immunogenic to induce antibody production and/or cellular immunity in the body and cause side effects, it is important for quality control to analyze these impurities. Monomers and dimers can be separated effectively using PROTEIN LW-803. Therefore, LW-803 is suitable for monitoring impurities.
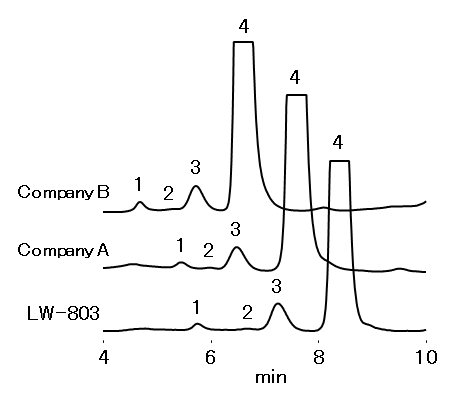
Sample : 20 μL
IgG from recombinant CHO cell 1 mg/mL
1. Aggregates
2. Trimer
3. Dimer
4. Monomer
| Resolution | ||
|---|---|---|
| monomer/dimer | dimer/trimer | |
| Company B | 2.1 | 2.6 |
| Company A | 2.5 | 2.4 |
| LW-803 | 2.6 | 3.4 |
Columns : Shodex PROTEIN LW-803 (8.0 mm I.D. x 300 mm)
Silica-based SEC column from other manufacturer (7.8 mm I.D. x 300 mm each)
Eluent : 50 mM Sodium phosphate buffer (pH7.0) + 0.3 M NaCl
Flow rate : 1.0 mL/min
Detector : UV (280 nm)
Column temp. : Room temp.
Sample Name Index
Operation Manual / Certificate of Analysis
Operation Manuals and Certificate of Analysis / Inspection Certificate for the following products can be downloaded here.
Product Name Index
Applications
- Sample Load (KW-803)
- Effects of Flow Rate (KW-802.5, KW-803)
- Standard Proteins (1) (KW-802.5)
- Standard Proteins (2) (KW-802.5 and KW-803)
- Standard Proteins (14) (KW-802.5)
- Standard Proteins (15) (KW-803)
- Standard Proteins (16) (KW-804)
- Standard Proteins (18) (KW-803)
- Standard Proteins (19) (KW-804)
- Standard Proteins (23) (GF-510 HQ)
- Standard Proteins (24) (KW402.5-4F)
- Standard Proteins (25) (KW403-4F)
- Standard Proteins (26) (KW404-4F)
- Standard Proteins (27) (KW405-4F)
- Recovery of Proteins (GF-310 HQ and GS-320 HQ)
- Recovery of Proteins (KW-802.5 and KW-803)
- Recovery of Proteins (KW-803)
- Proteins in Human Serum (3) (KW-803)
- Dimer and Trimer of BSA
- Crude Albumin (Chicken Egg) (1) (KW-803)
- Crude Lipoxidase
- Crude Catalase
- Crude Hexokinase
- Human Serum (1) (SB-802 HQ)
- Tryptic Digest of Human IgG
- Comparison of Chromatograms of Protein mixtures
- Comparison between KW402.5-4F and KW-802.5
- Recovery of Proteins (KW402.5-4F, KW403-4F)
- Human Control Serum
- Analysis of Whey in Yogurt (KW403 4F + KW402.5 4F)
- Lectin Protein
- Peptide Analysis (SEC)
- PEG-Chymotrypsin (KW-802.5)
- SEC Analysis of PEG-Asparaginase (SB-806 HQ)
- Human IgM (2)
- Poly-Lysine
- Avidin (2)
- α1 Acid Glycoprotein
- Analysis of Gelatin (SB-806M HQ)
- Analysis of Insulin Glargine According to JP Method (KW-802.5)
- Analysis of Human Insulin According to JP Method (KW-802.5)
- Analysis of High Molecular Impurities in Insulin Aspart According to Pharmacopeia Method (KW-802.5)
- Analysis of High Molecular Weight Protein According to USP-NF GENERAL CHAPTERS Physicochemical Analytical Procedures for Insulins (KW-802.5)
- Comparison of SEC Separation of Standard Proteins
- Comparison of SEC Separation of Polyclonal Antibody
- Comparison of Separation of BSA Aggregates (LW-803, KW-803)
- Monitoring Papain Digestion of Humanized Monoclonal IgG (LW-803)
- Analysis of Lipoproteins in Serum (KW-804)
- Comparison of SEC Separation of Standard Proteins between LW-403 4D and LW-803
- Durability Test by Number of Sample Injections (LW-403 4D)
- Analysis of Epoetin According to USP-NF Method (LW-803)
- Comparison of Separation of Proteins with Conventional Device and Semi-micro Type Device (LW-403 4D)
- Comparison of IgG Separation with Conventional Device and Semi-micro Type Device (LW-403 4D)
- Particle Size Distribution Determination of Hepatitis B Surface Antigen Virus-like Particle (HBsAg VLP) (KW405-4F)
- Analysis of Hemocyanin (KW405-4F)
- Analysis of Somatropin According to EP Method (LW-803)
- SEC Analysis of Rituximab (LW-803)
- SEC/MALS Analysis of Norovirus VLP (SB-805 HQ)
- Analysis of Thymosin α1 (KW-802.5)
- SEC/MALS Analysis of Exosome (SB-806 HQ)
- Analysis of Proteoglycan in a Supplement (SB-806M HQ)
- Molecular Weight Measurements of Low Molecular Weight Heparins According to USP-NF GENERAL CHAPTERS <209> (KW-803 + KW-802.5)
Applications (Related Information)
- Calibration Curves for KW-800 Series (Pullulan)
- Calibration Curves for KW-800 Series (Proteins)
- Calibration Curves for KW-800 Series (PEG, PEO)
- Calibration Curves of KW400-4F (1) (Pullulan)
- Calibration Curves of KW400-4F (2) (Proteins)
- Calibration Curves of KW400-4F (3) (PEG, PEO)
- Calibration Curves for GF-HQ Series (Aqueous Eluent: Proteins)
- Calibration Curve for LW-803 (Proteins)
- Calibration Curve for LW-403 4D (Proteins)
